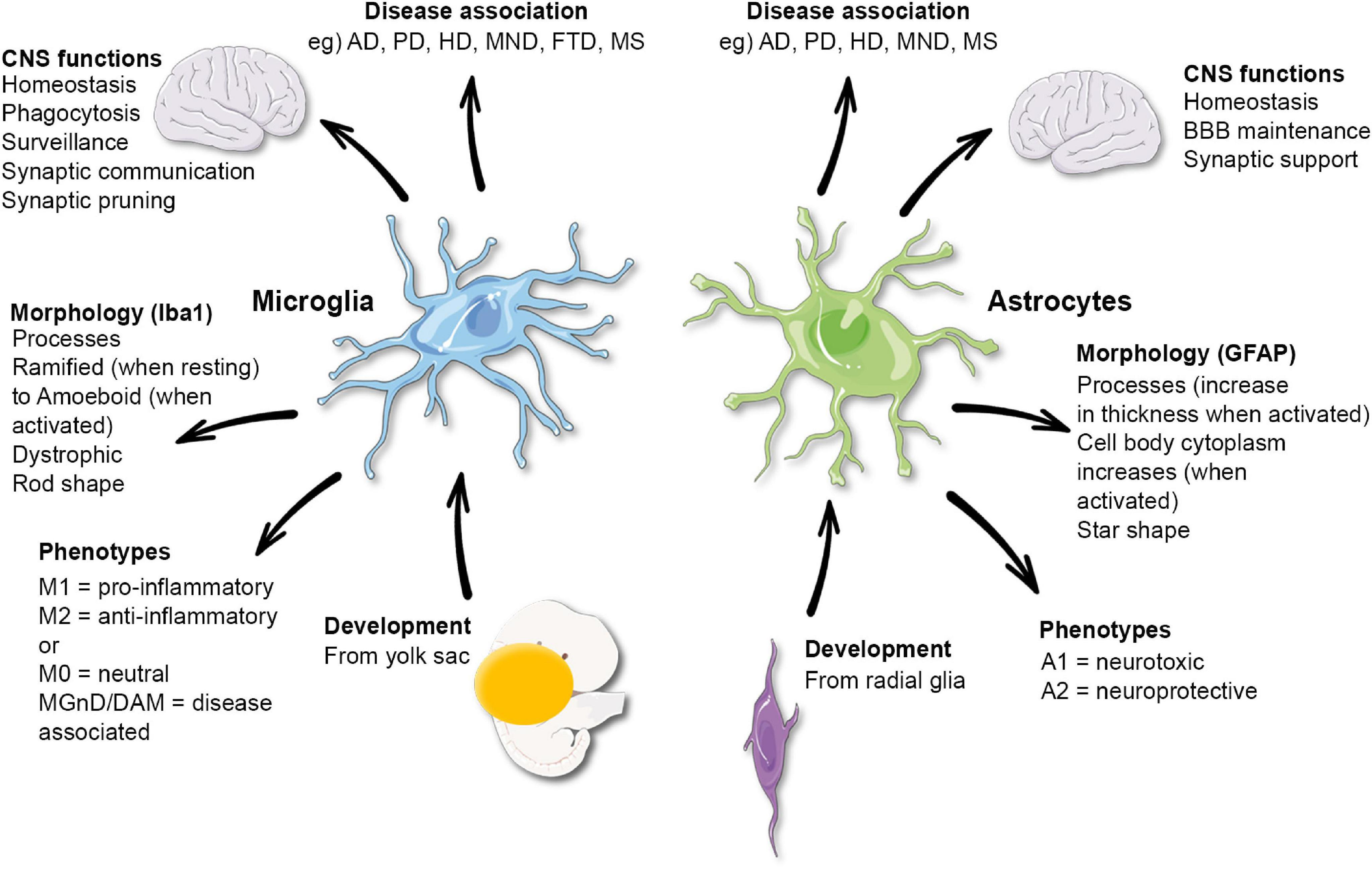
Microglial cells play a crucial role as the brain’s immune system, defending against injury and disease while actively participating in brain maintenance. These unique cells are essential in the context of Alzheimer’s research, where they help clear away cellular debris and prune synapses that transmit information among neurons. However, when the pruning process turns erroneous, it can contribute to the development of neurodegenerative diseases like Alzheimer’s and Huntington’s. Leading neuroscientist Beth Stevens has made significant strides in understanding how microglial dysfunction can wreak havoc on brain health, thus creating potential pathways for innovative therapies. Through her pioneering work at Boston Children’s Hospital and the Broad Institute, she is laying the groundwork for new biomarkers and treatment strategies aimed at ameliorating the impacts of these devastating diseases on millions of lives in America.
In the realm of neuroscience, microglia are often referred to as the brain’s guardians, performing vital functions similar to immune cells throughout the body. These protectors not only survey for cellular damage but also play an integral role in the intricate process known as synaptic pruning, which is essential for healthy brain development and function. Researchers, including notable figures like Beth Stevens, emphasize the importance of understanding these cells to unravel the complexities of various neurodegenerative disorders. By exploring the dynamics of the brain’s cellular ecosystem, scientists are uncovering new insights that could revolutionize Alzheimer’s treatment and improve the quality of life for those affected. The narrative surrounding microglia and their implications is thus foundational for advancing our knowledge about brain health and disease management.
Understanding Microglial Cells: The Brain’s Immune Defenders
Microglial cells are essential components of the brain’s immune system, playing a pivotal role in maintaining brain health. They act as the first line of defense against injury and disease, continuously monitoring the brain’s microenvironment for any signs of dysfunction or damage. When activated, microglia engage in several critical processes, including the removal of dead cells and the facilitation of synaptic pruning. This process allows the brain to maintain its optimal function by removing unnecessary or damaged synapses, an action crucial for neural plasticity and cognitive function.
Recent research led by renowned neuroscientist Beth Stevens has unveiled the complexities of microglial functions in the context of neurodegenerative diseases like Alzheimer’s. Stevens’ lab has highlighted how dysregulated microglial activity can lead to excessive synaptic pruning, which is associated with cognitive decline. By understanding microglia’s dual roles in both protection and potential harm, researchers are paving the way for novel therapeutic strategies aimed at restoring proper microglial function to combat diseases such as Alzheimer’s.
Linking Microglial Dysfunction to Neurodegenerative Diseases
The link between microglial dysfunction and neurodegenerative diseases has become an important area of focus in contemporary Alzheimer’s research. As the brain’s immune cells, microglia are tasked with detecting and responding to cellular damage. However, aberrant activity, such as inappropriate synaptic pruning, can exacerbate conditions like Alzheimer’s and Huntington’s diseases. This realization has led scientists to re-evaluate strategies for early intervention, emphasizing the importance of targeting microglial pathways in therapeutic development.
Beth Stevens’ groundbreaking research sheds light on how modifying microglial activity might protect against synapse loss and cognitive decline. By identifying biomarkers associated with unhealthy microglial behavior, Stevens and her team aspire to develop medications that restore balance to these immune cells. The implications of this research extend beyond Alzheimer’s, potentially impacting a broader spectrum of neurodegenerative diseases that share similar pathological features, reinforcing the critical need for research into the brain’s immune mechanisms.
The Role of Synaptic Pruning in Healthy Brain Function
Synaptic pruning is a natural process that occurs during brain development and continues throughout adulthood, with microglial cells playing a crucial role in its regulation. This process is essential for refining neural circuits and ensuring that the most effective connections between neurons are maintained. During early brain development, excessive synapses are formed, and pruning helps sculpt neural pathways as the brain matures. This precise regulation promotes cognitive efficiency and adaptability.
However, when synaptic pruning becomes dysregulated, as seen in various neurodegenerative diseases, it can lead to significant neuronal loss and functional impairments. Understanding the mechanisms underlying synaptic pruning, including the influence of microglial cells, is vital for developing targeted therapies. As researchers like Beth Stevens continue to dissect these processes, they aim to uncover the potential for therapeutic interventions that could prevent the detrimental effects of excessive or insufficient synaptic pruning in conditions such as Alzheimer’s.
Beth Stevens: A Pioneer in Neuroimmune Research
Beth Stevens, an esteemed neuroscientist and associate professor at Harvard Medical School, has emerged as a leading figure in the field of neuroimmune research, particularly in relation to Alzheimer’s and other neurodegenerative diseases. Her journey began with a fundamental curiosity about the brain’s immune system and its influence on synaptic connections. With significant support from the National Institutes of Health, Stevens has focused her efforts on understanding microglial cells and their essential roles in synaptic pruning and maintenance of neuronal health.
Her groundbreaking discoveries have not only expanded our understanding of how the brain copes with disease but also opened avenues for innovative therapeutic strategies. By elucidating the mechanisms through which microglial cells can either support or hinder cognitive function, Stevens has laid the groundwork for the development of potential interventions that may alleviate the impacts of diseases like Alzheimer’s, aiming to improve the quality of life for millions affected by these conditions.
Innovative Therapies Targeting the Brain’s Immune System
The pursuit of innovative therapies targeting the brain’s immune system is gaining momentum in the field of Alzheimer’s research. As scientists like Beth Stevens continue to uncover the intricacies of microglial cell functions, there is growing optimism about developing treatments that can modulate their activity. By addressing microglial dysfunction, researchers hope to mitigate the adverse effects associated with neurodegenerative diseases, paving the way for novel solutions that could restore synaptic health and cognitive function.
Recent advancements in drug development aim to harness microglial cells’ abilities to clear cellular debris and promote synaptic repair. These therapeutic strategies rely on understanding the signaling pathways that activate microglia and their role in synaptic pruning. By creating targeted interventions that promote healthy microglial function, scientists aspire to create a therapeutic landscape where the progression of Alzheimer’s and similar diseases is effectively slowed or even reversed, ultimately enhancing patient care and outcomes.
The Intersection of Basic Science and Clinical Research
The intersection of basic science and clinical research is crucial in the quest to understand and treat neurodegenerative diseases. Researchers like Beth Stevens exemplify how foundational science contributes to translational advancements. By exploring the functions and behaviors of microglial cells under various conditions, Stevens is helping bridge the gap between cellular mechanisms and clinical applications. These fundamental insights are essential for developing effective treatments that address the root causes of conditions like Alzheimer’s.
As studies on microglia evolve, the collaboration between basic and clinical researchers is more important than ever. This synergy fosters innovative approaches to treatment, enabling scientists to test hypotheses derived from basic research in clinical settings. Understanding how microglial dysregulation leads to neurodegeneration will ultimately guide the development of targeted therapies that can improve patient outcomes, making the case for continued investment in both realms of scientific inquiry.
The Significance of Federal Funding in Alzheimer’s Research
Federal funding has played a significant role in advancing Alzheimer’s research and exploring the functions of microglial cells. As Beth Stevens and her lab exemplify, support from the National Institutes of Health has been paramount in facilitating groundbreaking studies that address the complexities of neurodegenerative diseases. This funding not only allows researchers to pursue innovative ideas but also promotes collaboration and the sharing of knowledge, creating a robust framework for discovery.
In the rapidly evolving landscape of neuroscience, sustained governmental investment is essential for driving progress. It supports the exploration of the brain’s immune system, encouraging studies that can lead to new biomarkers and therapeutic approaches. By prioritizing funding for research initiatives focused on conditions like Alzheimer’s, policymakers can help ensure that scientific discoveries translate into meaningful improvements in diagnostics and treatment options for millions affected by these devastating diseases.
Future Directions in Neuroimmune Research
The future of neuroimmune research looks promising as more scientists recognize the critical role that microglial cells play in brain health and disease. Exploring the complexities of the brain’s immune system offers unique opportunities to uncover novel intervention strategies for Alzheimer’s and other neurodegenerative diseases. As researchers like Beth Stevens lead the charge in understanding how microglial dysregulation contributes to synaptic loss and cognitive decline, the field is poised for transformative breakthroughs.
By investigating the potential of microglial-modulating therapies, researchers aim to create a paradigm shift in how neurodegenerative diseases are approached. The integration of advanced technologies, such as genetic sequencing and imaging techniques, alongside traditional research methods, will continue to illuminate the intricate relationship between microglia and neuronal health. These efforts will ultimately pave the way for innovative treatments that target the immunological aspects of neurodegeneration, offering hope for patients and families affected by Alzheimer’s.
The Role of Biomarkers in Alzheimer’s Disease Detection
Biomarkers are increasingly recognized as a crucial element in the early detection and diagnosis of Alzheimer’s disease. By linking neuroimmune responses, such as those involving microglial cells, to specific biomarkers, researchers can improve the accuracy of Alzheimer’s diagnoses. Understanding the patterns of microglial behavior in the brain can lead to the identification of biomarkers that reflect not only the presence of neuroinflammation but also the degree of synaptic pruning that has occurred.
Beth Stevens’ contributions in identifying such biomarkers may advance the field of Alzheimer’s research, facilitating earlier interventions that can slow disease progression. As the search for reliable biomarkers continues, the hope is that these insights will enable clinicians to tailor treatments more effectively, ultimately enhancing patient care and improving outcomes for those grappling with Alzheimer’s disease.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial in Alzheimer’s research as they serve as the brain’s immune system, patrolling for signs of illness and injury. Their function includes clearing out dead or damaged cells and participating in synaptic pruning, a process that can, if functioning aberrantly, contribute to neurodegenerative diseases like Alzheimer’s.
How do microglial cells affect neurodegenerative diseases?
Microglial cells are implicated in various neurodegenerative diseases as they can become hyperactive or misfunction in their pruning role, potentially leading to neuronal damage. Understanding the role of microglia in these processes is vital for developing new treatments for conditions such as Alzheimer’s and Huntington’s disease.
What discovery did Beth Stevens make about microglial cells?
Beth Stevens’ research highlighted the dual role of microglial cells in both normal brain function and disease. She found that improper synaptic pruning by these cells could contribute to the development of Alzheimer’s and other neurodegenerative diseases, leading to potential new biomarkers and therapies.
What is synaptic pruning in relation to microglial cells?
Synaptic pruning is a process facilitated by microglial cells that involves the elimination of unnecessary synapses to enhance neural circuit efficiency. This process is essential during brain development and is vital for learning and memory; however, dysregulation can lead to neurodegenerative diseases like Alzheimer’s.
Why are microglial cells considered part of the brain’s immune system?
Microglial cells are termed the brain’s immune system because they monitor the central nervous system for signs of injury or infection. They respond by clearing damaged cells and debris and are involved in inflammatory responses that maintain brain health, which is critical in the context of neurodegenerative diseases.
What advancements have been made in Alzheimer’s research due to microglial studies?
Research on microglial cells has led to advancements in Alzheimer’s research by uncovering their role in synaptic pruning and neuroinflammation. This understanding has paved the way for developing new biomarkers and therapeutic strategies aimed at mitigating the effects of Alzheimer’s disease.
What funding supports research on microglial cells and Alzheimer’s?
Beth Stevens’ research on microglial cells and their role in Alzheimer’s disease has primarily been supported by the National Institutes of Health (NIH). This funding is crucial for advancing the science and translational research necessary to understand and combat neurodegenerative diseases.
How does studying microglial cells in mice benefit Alzheimer’s research?
Studying microglial cells in mice allows researchers to explore complex mechanisms that would be difficult to examine in humans. These investigations can reveal fundamental insights into how microglia function in health and disease, informing potential treatment approaches for Alzheimer’s and similar conditions.
| Key Point | |
|---|---|
| Microglial Cells Function | Microglial cells act as the brain’s immune system by patrolling for signs of illness or injury and clearing out dead or damaged cells. |
| Aberrant Pruning | Improper synaptic pruning by microglia can contribute to neurodegenerative diseases such as Alzheimer’s and Huntington’s. |
| Research Impact | Beth Stevens’ research has opened pathways for new biomarkers and treatments for Alzheimer’s disease. |
| Funding and Support | The research has been heavily supported by NIH funding, which has been vital for the progress of studies on microglial cells. |
| Importance of Basic Science | Basic science research, like that on microglia, is crucial for discovering new medical treatments and improving human health. |
Summary
Microglial cells play a critical role in maintaining brain health and are essential to understanding neurodegenerative diseases. Through their function as the brain’s immune response, they actively engage in clearing debris and regulating synaptic connections. Recent research demonstrates that when this process goes awry, it can contribute to debilitating conditions like Alzheimer’s disease. Therefore, the continued exploration of microglial cells is not just vital for scientific advancement; it is also a key component in the development of targeted therapies for millions affected by these conditions.