Gene Therapy for Hemophilia: A Breakthrough in Treatment
Gene therapy for hemophilia is making waves in the medical community as a groundbreaking solution for those living with this chronic condition. With the latest advancements, particularly the release of Hemgenix therapy, patients like Terence Blue are experiencing newfound hope and freedom from the daily burdens of traditional hemophilia management. This innovative approach leverages gene editing for hemophilia to address the underlying genetic cause of the disorder, potentially transforming lives by eliminating the need for regular clotting factor replacement. As studies show promising results, the focus now shifts toward accessibility and affordability of these cutting-edge treatments. The journey from complexity to simplicity in hemophilia care is not just a dream but a developing reality for many under this revolutionary therapy.
The emergence of advanced treatment options represents a significant leap in the realm of hemophilia care, commonly known as a bleeding disorder due to its impact on the blood’s ability to clot. Innovations in therapeutic approaches, including genetic therapies, allow for the correction of the underlying genetic flaws that lead to hemophilia B. This account of patients like Terence Blue exemplifies the shift towards a future where invasive procedures and regular treatments might gradually become relics of the past. By utilizing techniques such as gene editing for hemophilia, medical professionals aim to provide more effective management strategies and potentially permanent solutions. As the healthcare landscape evolves, the emphasis on therapies like Hemgenix continues to rise, promising significant enhancements in patients’ quality of life.
Understanding Gene Therapy for Hemophilia B
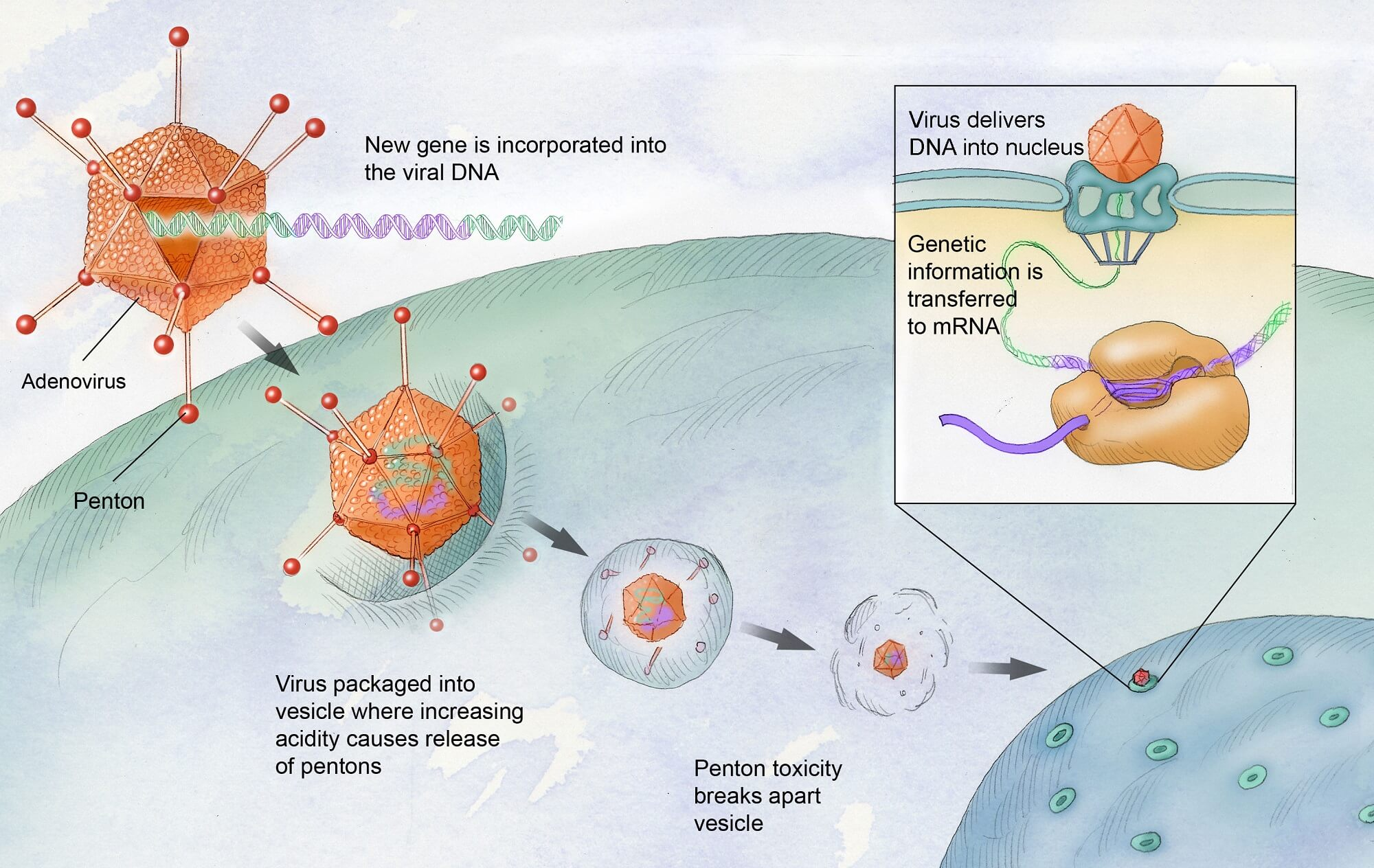
Gene therapy for hemophilia B, particularly the recently approved Hemgenix, represents a paradigm shift in the treatment landscape for patients suffering from this challenging condition. By utilizing a viral vector to deliver corrected genes directly into patients’ liver cells, Hemgenix aims to produce factor IX, the clotting protein that those with hemophilia B lack. Unlike traditional treatments that require regular injections of clotting factors, this gene therapy offers the potential for a long-term solution, reducing the burden of ongoing therapy and drastically improving patients’ quality of life.
The efficacy of gene therapy for hemophilia B has been notable, with clinical trials showing high success rates. Patients like Terence Blue, who received Hemgenix, have reported significant increases in their factor IX levels, which have led to fewer spontaneous bleeding episodes. This innovative approach not only promises reduced dependency on frequent infusions of clotting factors but also reshapes the future of hemophilia management. As researchers continue to refine these therapies and explore their applications across various genetic disorders, the hope is to eventually provide a full cure for hemophilia B and improve life expectancy for affected individuals.
The Impact of Hemgenix Therapy on Patients
Hemgenix therapy is generating immense excitement among patients and healthcare professionals alike. For individuals like Terence Blue, who have managed hemophilia their entire lives, the prospect of a single treatment that could alleviate the constant worry of bleeding episodes is transformative. The reduction in the need for regular clotting factor replacements can lead to a more active lifestyle, freeing patients from the limitations imposed by their condition. Experiences shared by patients post-treatment indicate not only improved physical health but also positive psychological effects, as they can engage in activities without fear of injury that could lead to severe complications.
However, while Hemgenix presents groundbreaking advancements, it doesn’t come without challenges. The high cost associated with these gene therapies raises questions about accessibility and insurance coverage. The experience from past gene therapy withdrawals highlights the importance of addressing economic factors to ensure that innovations like Hemgenix can benefit the patients who need them most. Nonetheless, the potential to dramatically change the lives of those with hemophilia B underscores the importance of ongoing research and advocacy for patient access to these transformative therapies.
Advancements in Hemophilia Management Techniques
Over the years, advancements in hemophilia management techniques have drastically improved the lives of many patients. Traditionally, the management of hemophilia involved regular injections of clotting factor replacement therapies, which helped to stave off bleeding episodes but could be inconvenient and painful. The introduction of longer-lasting synthetic factors has allowed patients to go longer between doses, but many still had to rely on consistent treatment routines. The rise of gene therapy, such as Hemgenix, represents an evolution in management techniques, providing the potential to treat the underlying genetic cause of hemophilia rather than merely managing its symptoms.
Innovations like prophylactic treatment strategies, which administer clotting factors before surgical procedures or anticipated bleeding events, have also played a crucial role in hemophilia management. These proactive approaches minimize the risk of spontaneous bleeding and help maintain joint health, particularly for young patients. As new therapies emerge, there is optimism that hemophilia management will continue to evolve, making life more manageable for patients and potentially enhancing their social interactions and overall quality of life.
The Role of Clotting Factor Replacement in Treatment
Clotting factor replacement remains a cornerstone in the treatment of hemophilia, despite the emergence of innovative therapies like gene therapy. For many patients, especially those without access to advanced treatments, regular injections of clotting factors are still necessary to prevent bleeding episodes. With the advancements in synthetic factor development, the composition and efficacy of these replacements have improved, allowing patients to lead more active lives. These treatments are particularly crucial for emergency situations or during serious physical activities where the risk of injury is higher.
Despite the progress, the reliance on clotting factor replacement highlights the urgent need for comprehensive hemophilia management strategies. Regular assessments by healthcare providers ensure that patients receive appropriate dosage adjustments and monitoring for potential complications. As the healthcare landscape evolves, incorporating gene therapies and integrating these with existing factor replacement protocols could present a holistic approach to hemophilia care, ultimately aiming for a future where patients may transition to fewer interventions and maintain healthier lives.
Challenges Faced in Gene Therapy Acceptance
While gene therapy offers remarkable potential for treating hemophilia B, several challenges in acceptance remain. Patients and healthcare providers must confront uncertainties regarding the long-term effects and overall safety of introducing gene therapies into the body. Given that Hemgenix targets the liver to induce factor IX production, patients like Terence Blue have had to weigh the potential benefits against the inherent risks of altering their genetic material. This process can be daunting, as it necessitates overcoming preconceived notions about gene editing and accepting a radical change to their treatment plan.
Furthermore, the high cost of gene therapies often deters patient acceptance, especially in a healthcare environment where insurance coverage may be limited. The substantial price tag associated with treatments like Hemgenix places an additional burden on already vulnerable populations living with hemophilia. It becomes incumbent upon healthcare systems, researchers, and policymakers to establish frameworks that facilitate patient access while addressing these economic barriers; thus ensuring that these groundbreaking therapies are available to all who can benefit from them.
Exploring Future Possibilities with Gene Editing
The future of hemophilia treatment may lie significantly in the realm of gene editing. Techniques like CRISPR offer promising pathways to directly correct genetic mutations that lead to hemophilia B, potentially paving the way toward true cures rather than symptom management. The possibility of editing genes to produce the functional factor IX naturally within the body holds transformative potential, dramatically changing the treatment paradigm for patients. This kind of innovative research is taking root in laboratories and institutions across the globe, setting the stage for revolutionary breakthroughs.
However, while the promise of gene editing is exciting, questions about its ethical implications, safety, and accessibility must be addressed. Ensuring that these advanced therapies are equitable and available to all patients, regardless of socioeconomic status, will be crucial as researchers press forward. The intersection of scientific inquiry and regulatory frameworks will play a pivotal role in shaping the future landscape of hemophilia treatment, allowing patients to benefit from both gene therapy and gene editing in ways we are only beginning to explore.
The Importance of Patient Education in Hemophilia Treatment
Patient education is an essential component of effective hemophilia management. Patients must be well-informed about their condition, potential treatment options, and the implications of newer therapies like Hemgenix. Understanding how gene therapy works can alleviate fears about potential side effects, making patients more willing to consider this innovative approach. Educational initiatives can empower patients with the knowledge needed to make informed decisions and collaborate effectively with their healthcare providers.
Moreover, improving awareness of hemophilia and its treatments among healthcare professionals is crucial. As gene therapies and advanced management techniques emerge, providers must be equipped with current information to guide their patients effectively. This ongoing education cycle not only enhances individual care but also fosters a supportive community for those living with hemophilia, ultimately improving treatment outcomes and quality of life.
Assessing Long-term Outcomes of Hemophilia Treatments
As the landscape of hemophilia treatment evolves, assessing the long-term outcomes of therapies like Hemgenix becomes paramount. Clinical trials have indicated that many patients experience significant improvements in their factor IX levels, reduced bleeding episodes, and enhanced quality of life post-treatment. However, the sustainability of these benefits over several years remains an area of active investigation. Understanding the durability of gene therapies will help inform clinical practices and guide expectations for both patients and healthcare professionals.
Furthermore, tracking the long-term effects on joint health and overall well-being among hemophilia patients who receive gene therapies is essential. Complications from chronic bleeding can result in arthritis and other joint issues, which significantly affect quality of life. As more patients like Terence Blue embark on their journey with gene therapy, ongoing studies will help establish comprehensive data to support the continued use and development of these groundbreaking treatments, ensuring that innovations lead to concrete long-term improvements.
The Evolution of Hemophilia Research: Past, Present, and Future
The field of hemophilia research has undergone significant shifts over the past few decades, evolving from mere management of symptoms to pursuing potential cures through gene therapy and innovative medical technology. The past saw patients relying heavily on clotting factor replacements, an approach that, while effective, was fraught with challenges. Today, with the approval of therapies like Hemgenix, the focus has shifted towards permanent solutions that aim to correct the genetic abnormalities responsible for hemophilia.
Looking ahead, the continued evolution in hemophilia research promises exciting possibilities, thanks to advances in gene editing and personalized medicine. As researchers strive to deepen their understanding of hemophilia’s complexity and the genetic engineering that could correct it, the hope remains that the future will bring about even more groundbreaking therapies. The collaborative efforts among scientists, healthcare providers, and patients will play a vital role in achieving the goal of entirely eradicating the challenges of living with hemophilia.
Frequently Asked Questions
What is gene therapy for hemophilia B and how does it work?
Gene therapy for hemophilia B, such as Hemgenix therapy, aims to provide a lasting solution by introducing a corrected version of the gene responsible for making clotting factor IX. This is achieved by using modified viruses to deliver the new gene into liver cells, where it can produce the clotting factor that patients are missing, significantly reducing or eliminating the need for traditional clotting factor replacement treatments.
How does Hemgenix therapy compare to traditional hemophilia B treatment options?
Hemgenix therapy represents a significant advancement in hemophilia B treatment. Unlike traditional hemophilia management, which often involves frequent injections of clotting factor replacement, Hemgenix aims to provide a long-term solution by enabling the body to produce its own clotting factor IX. This could reduce the burden of regular infusions and improve the quality of life for patients.
What are the potential benefits of gene editing for hemophilia management?
Gene editing for hemophilia management offers exciting potential benefits, including the possibility of a permanent fix to the genetic cause of hemophilia. By specifically targeting and repairing the defective gene responsible for clotting factor deficiencies, treatments like Hemgenix can significantly reduce or eliminate bleeding episodes and improve patients’ overall health outcomes.
Are there risks associated with gene therapy for hemophilia?
As with any medical treatment, gene therapy for hemophilia comes with potential risks, such as unexpected immune responses or changes in liver function. However, clinical trials of Hemgenix have shown promising results with few severe side effects, suggesting that the benefits may outweigh the risks for many patients. Ongoing monitoring and study will help determine the long-term safety of these therapies.
How much does Hemgenix therapy cost and is it covered by insurance?
The list price for Hemgenix therapy is approximately $3.5 million. However, many insurance companies negotiate lower rates, and coverage typically depends on individual plans and patient circumstances. Patients should consult with their healthcare provider and insurance provider to understand coverage options for this gene therapy.
What results can patients expect after receiving gene therapy for hemophilia B?
Patients receiving gene therapy for hemophilia B, such as Hemgenix, can expect a significant increase in their clotting factor IX levels, leading to fewer or even no bleeding episodes. Clinical trials have demonstrated that a majority of treated patients require no prophylactic clotting factor replacements three years post-treatment, indicating the potential for long-lasting effects.
Who is eligible for gene therapy for hemophilia, like Hemgenix?
Eligibility for gene therapy for hemophilia, including Hemgenix, typically includes individuals diagnosed with hemophilia B who have severe symptoms or those not adequately managed by traditional treatments. Factors such as age, liver health, and overall medical history will be considered by healthcare providers to determine suitability.
How does gene therapy for hemophilia impact the daily lives of patients?
Gene therapy for hemophilia has the potential to significantly improve the daily lives of patients by reducing their reliance on regular clotting factor injections. This could enable greater freedom in activities, reduce hospital visits, and lessen anxiety associated with bleeding episodes, improving overall quality of life.
| Key Points | Details |
|---|---|
| Gene Therapy Introduction | Terence Blue became the first patient in New England to receive Hemgenix, a gene therapy for hemophilia B. |
| Treatment Background | Developed by CSL Behring, Hemgenix aims to treat hemophilia B by introducing corrected genes to produce necessary clotting factors. |
| Market Challenges | High costs (e.g. $3.5 million for Hemgenix) and inadequate patient interest could impact the sustainability of gene therapy options. |
| Treatment Process | Used a virus to target the liver and insert a corrected copy of the gene causing hemophilia B. |
| Patient Experience | Patients like Blue experience significant lifestyle changes and report fast healing after treatment. |
| Outcomes | Initial results show many patients no longer require factor IX prophylaxis three years post-treatment. |
| Future of Gene Therapy | Ongoing research and advancements illustrate hope for more effective and accessible treatments. |
Summary
Gene therapy for hemophilia has made significant strides with innovative treatments like Hemgenix. This breakthrough not only alleviates the constant worry of bleeding but also reduces the need for frequent injections. As the field of gene therapy continues to evolve, patients are finding hope and improved quality of life through these advanced medical interventions.
You may also like
Archives
Calendar
| M | T | W | T | F | S | S |
|---|---|---|---|---|---|---|
| 1 | ||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| 16 | 17 | 18 | 19 | 20 | 21 | 22 |
| 23 | 24 | 25 | 26 | 27 | 28 | 29 |
| 30 | 31 | |||||